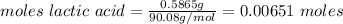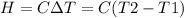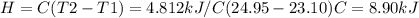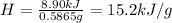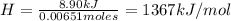Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1
You know the right answer?
A0.5865-g sample of lactic acid (hc3h5o3) is burned in a calorimeter whose heat capacity is 4.812 kj...
Questions

Biology, 13.03.2020 03:27

Mathematics, 13.03.2020 03:27

Mathematics, 13.03.2020 03:27

Mathematics, 13.03.2020 03:27





Mathematics, 13.03.2020 03:27

Mathematics, 13.03.2020 03:27

Advanced Placement (AP), 13.03.2020 03:27



Geography, 13.03.2020 03:27

Mathematics, 13.03.2020 03:27



Biology, 13.03.2020 03:27