Chemistry, 05.09.2019 20:20 kuehnkeegan
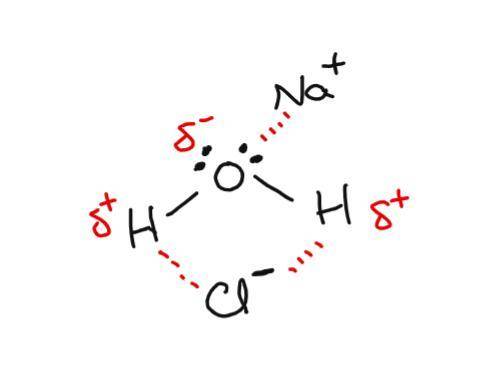
Awater molecule has polar o−h bonds that result in regions of partial positive charge (hydrogen atoms) and a region of partial negative charge (oxygen atom with lone pairs). place the na+ and cl− ions where h2o molecules are properly oriented to form ion–dipole interactions.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2


Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
Awater molecule has polar o−h bonds that result in regions of partial positive charge (hydrogen atom...
Questions


Mathematics, 15.10.2020 01:01

Mathematics, 15.10.2020 01:01


Mathematics, 15.10.2020 01:01


Social Studies, 15.10.2020 01:01

Biology, 15.10.2020 01:01


Mathematics, 15.10.2020 01:01

Physics, 15.10.2020 01:01

Mathematics, 15.10.2020 01:01




Chemistry, 15.10.2020 01:01


Computers and Technology, 15.10.2020 01:01

History, 15.10.2020 01:01

Mathematics, 15.10.2020 01:01