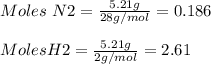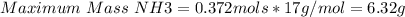Chemistry, 05.09.2019 21:30 tasnimsas3
For the following reaction, 5.21 grams of nitrogen gas are allowed to react with 5.91 grams of hydrogen gas.
nitrogen (g) + hydrogen (g) → ammonia (g)
what is the maximum amount of ammonia that can be formed?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
You know the right answer?
For the following reaction, 5.21 grams of nitrogen gas are allowed to react with 5.91 grams of hydro...
Questions



History, 16.11.2020 18:50

Mathematics, 16.11.2020 18:50

Mathematics, 16.11.2020 18:50

Physics, 16.11.2020 18:50

Arts, 16.11.2020 18:50



Spanish, 16.11.2020 18:50



Mathematics, 16.11.2020 18:50


Mathematics, 16.11.2020 18:50

Biology, 16.11.2020 18:50

Mathematics, 16.11.2020 18:50

Health, 16.11.2020 18:50