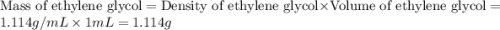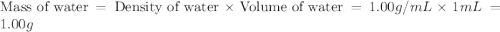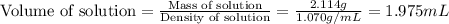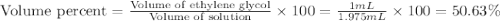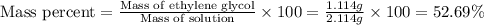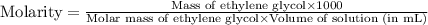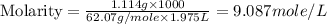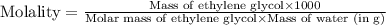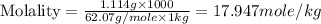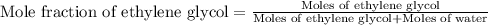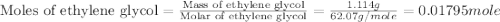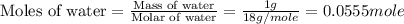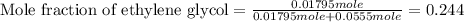An automobile antifreeze mixture is made by mixing equal volumes of ethylene glycol (d = 1.114 g/ml; m = 62.07 g/mol) and water (d = 1.00 g/ml) at 20°c. the density of the mixture is 1.070 g/ml. express the concentration of ethylene glycol as
a- volume percent
b- mass percent
c- molarity
d- molality
e- mole fraction

Answers: 1
Another question on Chemistry



Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1
You know the right answer?
An automobile antifreeze mixture is made by mixing equal volumes of ethylene glycol (d = 1.114 g/ml;...
Questions



English, 24.09.2019 19:10





Mathematics, 24.09.2019 19:10

Biology, 24.09.2019 19:10

History, 24.09.2019 19:10


English, 24.09.2019 19:10

Mathematics, 24.09.2019 19:10

Computers and Technology, 24.09.2019 19:10

English, 24.09.2019 19:10

Mathematics, 24.09.2019 19:10

Biology, 24.09.2019 19:10

English, 24.09.2019 19:10


English, 24.09.2019 19:10