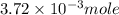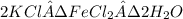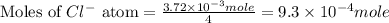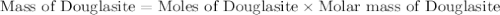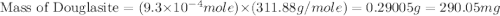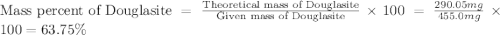Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Douglasite is a mineral with the formula 2kcl # fecl2 # 2h2o. calculate the mass percent of douglasi...
Questions

Mathematics, 16.01.2020 12:31

Mathematics, 16.01.2020 12:31

Chemistry, 16.01.2020 12:31


World Languages, 16.01.2020 12:31




Mathematics, 16.01.2020 12:31

Biology, 16.01.2020 12:31


History, 16.01.2020 12:31


History, 16.01.2020 12:31




Business, 16.01.2020 12:31


Chemistry, 16.01.2020 12:31

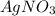 .
.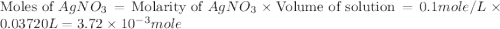
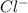 =
=