
Chemistry, 05.09.2019 23:10 portedon6626
Write qc for each of the following: (1) gaseous sulfur tetrafluoride reacts with liquid water to produce gaseous sulfur dioxide and hydrogen fluoride gas. (2) solid molybdenum(vi) oxide reacts with gaseous xenon difluoride to form liquid molybdenum(vi) fluoride, xenon gas, and oxygen gas.

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
Write qc for each of the following: (1) gaseous sulfur tetrafluoride reacts with liquid water to pro...
Questions

Mathematics, 05.08.2021 01:00

Mathematics, 05.08.2021 01:00





History, 05.08.2021 01:00

Mathematics, 05.08.2021 01:00



Computers and Technology, 05.08.2021 01:00







Chemistry, 05.08.2021 01:00


![Q_c=\frac{[SO_2][HF]^4}{[SF_4]}](/tpl/images/0223/9095/5d98b.png)
![Q_c=\frac{[O_2]^2[Xe]}{[XeF_2]}](/tpl/images/0223/9095/5b7c4.png)
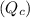 : It is defined as the measurement of the relative amounts of products and reactants present during a reaction at a particular time.
: It is defined as the measurement of the relative amounts of products and reactants present during a reaction at a particular time.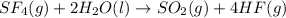
![2MoO_2(s)+XeF_2(g)\rightarrow 2MoF(l)+Xe(g)+2O_2(g)[/texIn this expression, only gaseous or aqueous states are includes and pure liquid or solid states are omitted. So, the expression for reaction quotient will be :[tex]Q_c=\frac{[O_2]^2[Xe]}{[XeF_2]}](/tpl/images/0223/9095/8e63c.png)


