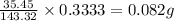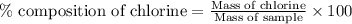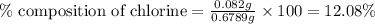The percent chloride in an unknown sample may be determined by gravimetric methods. suppose a 0.6789g sample of an unknown chloride sample was dissolved and agcl is precipitated by adding agno3 solution. the precipitate was filtered, ignited, and found to weigh 0.g. what was the percent chloride in the sample?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
The percent chloride in an unknown sample may be determined by gravimetric methods. suppose a 0.6789...
Questions


Medicine, 19.02.2021 05:00

Mathematics, 19.02.2021 05:00






Mathematics, 19.02.2021 05:00


English, 19.02.2021 05:00




Mathematics, 19.02.2021 05:00