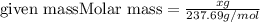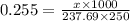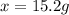Chemistry, 06.09.2019 04:10 valeriekbueno
How many grams of nickel (ii) chloride hexahydrate are required to prepare 250. ml of aqueous solution whose concentration is 0.255 m? the molar mass of nickel (ii) chloride hexahydrate is 237.69 g mol−1.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
How many grams of nickel (ii) chloride hexahydrate are required to prepare 250. ml of aqueous soluti...
Questions


History, 08.12.2020 01:00



English, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00







Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

Social Studies, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

Arts, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

Social Studies, 08.12.2020 01:00

History, 08.12.2020 01:00

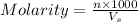
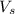 = volume of solution in ml
= volume of solution in ml