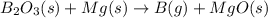B)elemental boron (as a gas) is produced in one industrial process by heating diboron trioxide with magnesium metal, also producing magnesium oxide as a by-product. write the unbalanced chemical equation for this process. (omit states-of-matter from your answer.)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
B)elemental boron (as a gas) is produced in one industrial process by heating diboron trioxide with...
Questions

Social Studies, 20.10.2021 14:00


English, 20.10.2021 14:00




Social Studies, 20.10.2021 14:00



Mathematics, 20.10.2021 14:00

Mathematics, 20.10.2021 14:00

History, 20.10.2021 14:00

Mathematics, 20.10.2021 14:00


Social Studies, 20.10.2021 14:00

Mathematics, 20.10.2021 14:00


Physics, 20.10.2021 14:00

Mathematics, 20.10.2021 14:00