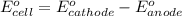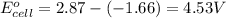Chemistry, 06.09.2019 16:20 glizbethh00
For the following electrochemical reaction: al3+(aq) + 3e -> al(s) eº = -1.66 v e° = 2.87 f2(g) + 2e -> 2f (aq) calculate eº for the cell.

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
For the following electrochemical reaction: al3+(aq) + 3e -> al(s) eº = -1.66 v e° = 2.87 f2(g)...
Questions


Mathematics, 25.06.2019 02:00

Biology, 25.06.2019 02:00

History, 25.06.2019 02:00


Mathematics, 25.06.2019 02:00


Mathematics, 25.06.2019 02:00

Mathematics, 25.06.2019 02:00

Spanish, 25.06.2019 02:00


English, 25.06.2019 02:00


Mathematics, 25.06.2019 02:00


Mathematics, 25.06.2019 02:00


Mathematics, 25.06.2019 02:00


History, 25.06.2019 02:00

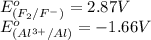
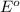 potential will always get reduced and will undergo reduction reaction. Here, fluorine will undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, fluorine will undergo reduction reaction will get reduced.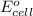 of the reaction, we use the equation:
of the reaction, we use the equation: