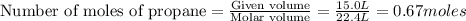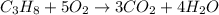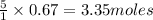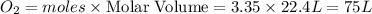Saved propane burns in air according to the equation c3ha(g 502lg)3co2) + 4h20(g) what volume of o2 in liters would be required if 15.0 l of propane burns, assuming that all of the gases are under the same conditions? short answer toolbar navigation e i e b ius ea this question will be sent to your instructor for grading. 20 of 25 l next > prev nere to search

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2



Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
Saved propane burns in air according to the equation c3ha(g 502lg)3co2) + 4h20(g) what volume of o2...
Questions




Medicine, 04.08.2021 23:30


Mathematics, 04.08.2021 23:30


Mathematics, 04.08.2021 23:30

Social Studies, 04.08.2021 23:30

Mathematics, 04.08.2021 23:30

Mathematics, 04.08.2021 23:30

History, 04.08.2021 23:30

History, 04.08.2021 23:30

Mathematics, 04.08.2021 23:30

English, 04.08.2021 23:30

Mathematics, 04.08.2021 23:30


Health, 04.08.2021 23:30

Chemistry, 04.08.2021 23:30

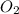 in liters would be required if 15.0 L of propane burns, assuming that all of the gases are under the same conditions.
in liters would be required if 15.0 L of propane burns, assuming that all of the gases are under the same conditions.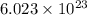 of particles.
of particles.