Chemistry, 06.09.2019 17:20 aaliyahbaladez56
Aluminum chloride is named as if it is ionic but it is really molecular, with the formula al2cl6. it can be formed by direct reaction of the elements. 2al(s) + 3cl2(g) → aicia) if 0.824 g of aluminum react with excess chlorine, how many grams of aluminum chloride can be obtained? (4.07 g) calculate the grams of chlorine that react with 0.194 g of aluminum. (0.765 g) 1.2.b

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
Aluminum chloride is named as if it is ionic but it is really molecular, with the formula al2cl6. it...
Questions



English, 04.02.2021 21:10

Social Studies, 04.02.2021 21:10


Mathematics, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10

Chemistry, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10


Chemistry, 04.02.2021 21:10





Mathematics, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10

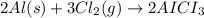
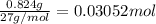
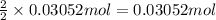 of aluminium chloride.
of aluminium chloride.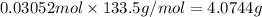
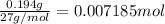
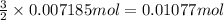 of chlorine gas.
of chlorine gas.