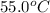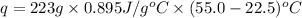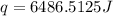Chemistry, 06.09.2019 17:30 PONBallfordM89
How much heat energy would be needed to raise the temperature of a 223 g sample of aluminum [(c=0.895 jig cy from 22.5°c to 55 0°c? η ο ο ο οο 10x10) not enough information given prov 40 25 11 next >

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3


Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
How much heat energy would be needed to raise the temperature of a 223 g sample of aluminum [(c=0.89...
Questions




Social Studies, 05.06.2021 03:30

Physics, 05.06.2021 03:30


Mathematics, 05.06.2021 03:30

Mathematics, 05.06.2021 03:30

Biology, 05.06.2021 03:30






Mathematics, 05.06.2021 03:30

Mathematics, 05.06.2021 03:30

Mathematics, 05.06.2021 03:30

Biology, 05.06.2021 03:30


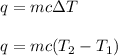
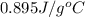
 = change in temperature
= change in temperature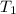 = initial temperature =
= initial temperature = 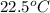
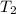 = final temperature =
= final temperature =