
Chemistry, 06.09.2019 17:30 wannamakerdaandre
Consider the general form of a reversible reaction to be: where the double arrow means the reaction can proceed in both the forward and reverse direction. if the equilibrium constant for the forward reaction is defined as: -, what is the equilibrium constant for the reverse reaction? a) it is exactly the same. namely b) it is just the products of the reactants. namely, c) it is equal to the reciprocal of the forward equilibrium constant. that is d) the reverse equilibrium constant cannot be determined. a) bi od) cl

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
Consider the general form of a reversible reaction to be: where the double arrow means the reaction...
Questions



Mathematics, 06.11.2020 17:10



Biology, 06.11.2020 17:10

English, 06.11.2020 17:10









Advanced Placement (AP), 06.11.2020 17:10




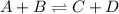
![K_{f} = \frac{[C][D]}{[A][B]}](/tpl/images/0224/4830/c1e46.png) .......... (1)
.......... (1)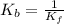
![\frac{[A][B]}{[C][D]}](/tpl/images/0224/4830/199c2.png) ............ (2)
............ (2)


