
Chemistry, 06.09.2019 18:10 itsyagirl11076
For the overall reaction below, which of the following is the correctly written rate law? overall reaction: o3(g)+2no2(g)→n2o5(g)+o2(g) step 1: o3(g)+no2(g)→no3(g)+o2(g) slow step 2: no3(g)+no2(g)→n2o5(g) fast view available hint(s) for the overall reaction below, which of the following is the correctly written rate law? overall reaction: step 1: slow step 2: fast rate=k[o3][no2]2 rate=k[no3][no2] rate=k[o3][no2] rate=k[o3][no2]2[n2o5][o2]

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
For the overall reaction below, which of the following is the correctly written rate law? overall r...
Questions



Biology, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01



Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01


History, 02.10.2020 14:01




Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Biology, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

![Rate=k[O_3][NO_2]^2](/tpl/images/0224/5146/9d0a3.png)
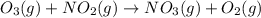 slow
slow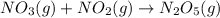 fast
fast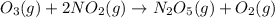
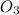 is 1 and Order with respect to
is 1 and Order with respect to 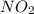 is 2.
is 2.

