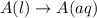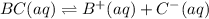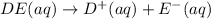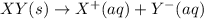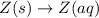Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
Each of the following reactions shows a solute dissolved in water. classify each solute as a strong...
Questions

Computers and Technology, 30.11.2020 17:40


Computers and Technology, 30.11.2020 17:40

Mathematics, 30.11.2020 17:40



Social Studies, 30.11.2020 17:40

Advanced Placement (AP), 30.11.2020 17:40

Mathematics, 30.11.2020 17:40




Health, 30.11.2020 17:40