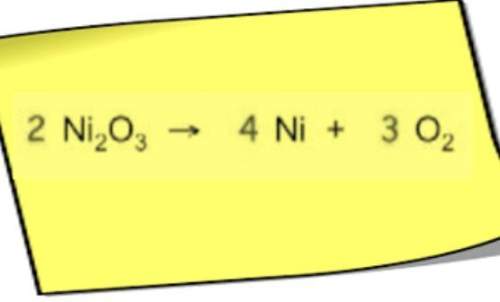
Chemistry, 06.09.2019 20:30 MayFlowers
Define a salt bridge. define a salt bridge. a pathway between the cathode and anode in which ions are oxidized. a pathway, composed of salt water, that ions pass through. a pathway between the cathode and anode in which ions are reduced. a pathway by which counterions can flow between the half-cells without the solutions in the half-cell totally mixing. a pathway in which no ions flow.

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
Define a salt bridge. define a salt bridge. a pathway between the cathode and anode in which ions ar...
Questions

Mathematics, 18.05.2021 14:00



Social Studies, 18.05.2021 14:00

Business, 18.05.2021 14:00


Mathematics, 18.05.2021 14:00


Computers and Technology, 18.05.2021 14:00





Mathematics, 18.05.2021 14:00

Computers and Technology, 18.05.2021 14:00

History, 18.05.2021 14:00



English, 18.05.2021 14:00