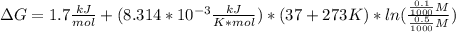Chemistry, 06.09.2019 20:30 angellynn581
2-phosphoglycerate(2pg) is converted to phosphoenolpyruvate (pep) by the enzyme enolase. the standard free energy change(deltago’) for this reaction is +1.7 kj/mol. if the cellular concentrations are 2pg = 0.5 mm and pep = 0.1 mm, what is the free energy change at 37 oc for the reaction 2pg ↔ pep
5.8 kj/mol
-5.8 kj/mol
+2.4 kj/mol
-2.4 kj/mol
-4146.4 kj/mol
+4146.4 kj/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
2-phosphoglycerate(2pg) is converted to phosphoenolpyruvate (pep) by the enzyme enolase. the standar...
Questions



History, 13.09.2019 22:10


Social Studies, 13.09.2019 22:10

Mathematics, 13.09.2019 22:10

History, 13.09.2019 22:10

Chemistry, 13.09.2019 22:10


Mathematics, 13.09.2019 22:10


Mathematics, 13.09.2019 22:10


Biology, 13.09.2019 22:10





Medicine, 13.09.2019 22:10

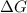 to
to 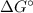 according to:
according to:![\Delta G=\Delta G\°+RTln(\frac{[products]^n}{[reagents]^m})](/tpl/images/0224/6819/0c5cf.png)