
Chemistry, 06.09.2019 21:20 villafana36
The complete oxidation of one mole of a sugar produces carbon dioxide and water. 2000 kj of heat is transferred from the system to the surroundings. the rearrangement of bonds as 0.5 moles of the sugar are oxidized generates heat in an open test tube (101 j•l–1 pressure and 300 k temperature). what is the change in internal energy of the system (δu)? what is the change in enthalpy of the system(δh)?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

You know the right answer?
The complete oxidation of one mole of a sugar produces carbon dioxide and water. 2000 kj of heat is...
Questions




Spanish, 24.04.2020 03:24


History, 24.04.2020 03:25



Mathematics, 24.04.2020 03:25

Mathematics, 24.04.2020 03:25

English, 24.04.2020 03:25

Mathematics, 24.04.2020 03:25


Mathematics, 24.04.2020 03:25

English, 24.04.2020 03:25



Mathematics, 24.04.2020 03:25


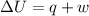
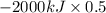
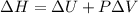

 ) is -1000 kJ.
) is -1000 kJ.

