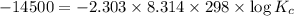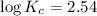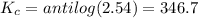Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1
You know the right answer?
Areaction has a standard free-energy change of -14.50 kj mol(-3.466 kcal mol). calculate the equilib...
Questions


Mathematics, 01.03.2021 18:20


History, 01.03.2021 18:20


Social Studies, 01.03.2021 18:20


English, 01.03.2021 18:20

Spanish, 01.03.2021 18:20



Mathematics, 01.03.2021 18:20



Mathematics, 01.03.2021 18:20


English, 01.03.2021 18:20



History, 01.03.2021 18:20

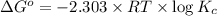
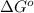 = standard Gibb's free energy change = -14.50kJ/mol =14500 J/mol
= standard Gibb's free energy change = -14.50kJ/mol =14500 J/mol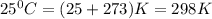
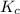 = equilibrium constant = ?
= equilibrium constant = ?