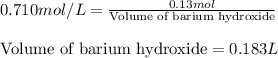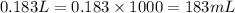Chemistry, 06.09.2019 22:10 zmoore8015
Calculate the number of milliliters of 0.710 m ba(oh)2 required to precipitate all of the mn2+ ions in 161 ml of 0.796 m kmno4 solution as mn(oh)2. the equation for the reaction is: mnso4(aq) + ba(oh)2(aq) mn(oh)2(s) + baso4(aq)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
Calculate the number of milliliters of 0.710 m ba(oh)2 required to precipitate all of the mn2+ ions...
Questions

Biology, 24.11.2020 21:40

Mathematics, 24.11.2020 21:40

Social Studies, 24.11.2020 21:40


Mathematics, 24.11.2020 21:40

Biology, 24.11.2020 21:40

Mathematics, 24.11.2020 21:40


Mathematics, 24.11.2020 21:40

Mathematics, 24.11.2020 21:40

History, 24.11.2020 21:40

Chemistry, 24.11.2020 21:40


Social Studies, 24.11.2020 21:40


History, 24.11.2020 21:40

Biology, 24.11.2020 21:40

Mathematics, 24.11.2020 21:40


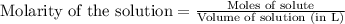 .....(1)
.....(1)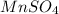 = 0.796 M
= 0.796 M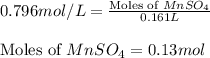
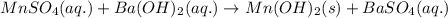
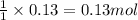 of barium hydroxide
of barium hydroxide