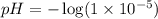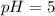Acid & base calculations calculate the hydronium ion concentration for each. tell whether it is an acid or a base. 1. ph = 5.54 2. poh = 9.7 3. ph = 7.0 4. ph = 12.9 5. poh = 1.2 calculate the poh for each. tell whether it is an acid or a base. 6. [h3o+] = 1 x 10-5 m

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
Acid & base calculations calculate the hydronium ion concentration for each. tell whether it is...
Questions

Social Studies, 19.10.2020 08:01

Mathematics, 19.10.2020 08:01

History, 19.10.2020 08:01




Computers and Technology, 19.10.2020 08:01

English, 19.10.2020 08:01



Mathematics, 19.10.2020 08:01


Mathematics, 19.10.2020 08:01

History, 19.10.2020 08:01


Arts, 19.10.2020 08:01

Mathematics, 19.10.2020 08:01

Mathematics, 19.10.2020 08:01


![pH=-\log[H_3O^+]](/tpl/images/0224/7714/a23b5.png) ......(1)
......(1)![5.54=-\log[H_3O^+]](/tpl/images/0224/7714/46ae8.png)
![[H_3O^+]=2.88\times 10^{-6}M](/tpl/images/0224/7714/94865.png)
![4.3=-\log[H_3O^+]](/tpl/images/0224/7714/cd75a.png)
![[H_3O^+]=5.012\times 10^{-5}M](/tpl/images/0224/7714/9980d.png)
![7.0=-\log[H_3O^+]](/tpl/images/0224/7714/bd612.png)
![[H_3O^+]=1.00\times 10^{-7}M](/tpl/images/0224/7714/e9a37.png)
![12.9=-\log[H_3O^+]](/tpl/images/0224/7714/250d5.png)
![[H_3O^+]=1.26\times 10^{-13}M](/tpl/images/0224/7714/8cabb.png)
![12.8=-\log[H_3O^+]](/tpl/images/0224/7714/d6d68.png)
![[H_3O^+]=1.58\times 10^{-13}M](/tpl/images/0224/7714/c23c1.png)
![[H_3O^+]=1\times 10^{-5}M](/tpl/images/0224/7714/3d88c.png)