
Chemistry, 06.09.2019 23:10 Mynameismath
The literature value of the atomic mass of an isotope of nickel is 57.9 g/mol. if a laboratory experimenter determined the mass to be 59.6 g/mol, what is the percent error?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
The literature value of the atomic mass of an isotope of nickel is 57.9 g/mol. if a laboratory exper...
Questions


English, 16.09.2019 08:30

History, 16.09.2019 08:30



Physics, 16.09.2019 08:30

Biology, 16.09.2019 08:30



Mathematics, 16.09.2019 08:30

Chemistry, 16.09.2019 08:30



Mathematics, 16.09.2019 08:50


History, 16.09.2019 08:50


History, 16.09.2019 08:50

Social Studies, 16.09.2019 08:50

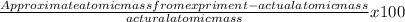
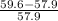 x 100 = 17%
x 100 = 17%


