
Chemistry, 07.09.2019 01:10 jiedwards3835
For the reaction pc15 (8) pc13 (g) + cl2 (g) k = 0.0454 at 261 °c. if a vessel is filled with these gases such that the initial concentrations are [pc15) = 0.20 m, [pc13] = 0.20 m, and (cl21 = 2.5 m, in which direction will a reaction occur and why? a) toward products because qc = 0.56 b) toward reactants because qc = 2.5 c) toward products because qc = 2.8 d) toward reactants because qc = 0.0454 e) it is at equilibrium because qc = 1

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
For the reaction pc15 (8) pc13 (g) + cl2 (g) k = 0.0454 at 261 °c. if a vessel is filled with these...
Questions



English, 11.04.2021 14:00

Social Studies, 11.04.2021 14:00

English, 11.04.2021 14:00



English, 11.04.2021 14:00


English, 11.04.2021 14:00


Chemistry, 11.04.2021 14:00

English, 11.04.2021 14:00





Mathematics, 11.04.2021 14:00

Social Studies, 11.04.2021 14:00

Mathematics, 11.04.2021 14:00

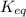 is samller than
is samller than 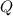 of the reaction . So,the reaction will shift towards the left i.e. towards the reactant side.
of the reaction . So,the reaction will shift towards the left i.e. towards the reactant side.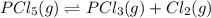
![Q=\frac{[PCl_3][Cl_2]}{[[PCl_5]^1}](/tpl/images/0224/9376/2eff0.png)
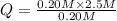
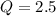
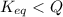 , the reaction will shift towards the left i.e. towards the reactant side.
, the reaction will shift towards the left i.e. towards the reactant side.

