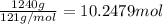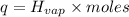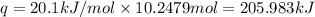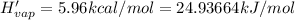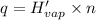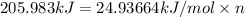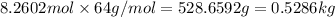Chemistry, 07.09.2019 01:30 krishimotam
Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 5.96 kcal/mol) was used in household refrigerators. what mass (in kg) of so2 must be evaporated to remove as much heat as evaporation of 1.24 kg of ccl2f2 (enthalpy of vaporization is 20.1 kj/mol)?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

You know the right answer?
Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 5.96 kcal/...
Questions



Mathematics, 05.09.2020 04:01



Computers and Technology, 05.09.2020 04:01


Mathematics, 05.09.2020 04:01


Mathematics, 05.09.2020 04:01

Mathematics, 05.09.2020 04:01





History, 05.09.2020 04:01


Mathematics, 05.09.2020 04:01


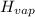 = 20.1 kJ/mol
= 20.1 kJ/mol