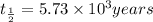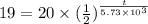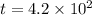Chemistry, 07.09.2019 02:30 castiaulii16
The half life for the decay of carbon-14 is 5.73 x 10 years. suppose the activity due to the radioactive decay of the carbon-14 in a tiny sample of an artifact made of wood from an archeological dig is measured to be 19. bq. the activity in a similar-sized sample of fresh wood is measured to be 20. bq. calculate the age of the artifact. round your answer to 2 significant digits. years x 5 ?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
The half life for the decay of carbon-14 is 5.73 x 10 years. suppose the activity due to the radioac...
Questions

Biology, 13.11.2020 14:00

Mathematics, 13.11.2020 14:00




Mathematics, 13.11.2020 14:00


Mathematics, 13.11.2020 14:00

Health, 13.11.2020 14:00


English, 13.11.2020 14:00



Mathematics, 13.11.2020 14:00

Mathematics, 13.11.2020 14:00





 years
years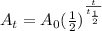
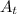 represents activity of radioactive nuclide after t time,
represents activity of radioactive nuclide after t time, 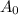 represents initial activity of radioactive nuclide and
represents initial activity of radioactive nuclide and 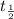 represents half-lifeHere,
represents half-lifeHere, 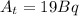 ,
, 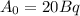 and
and