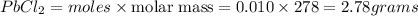Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

You know the right answer?
What is the theoretical yield (in g of precipitate) when 16.3 ml of a 0.628 m solution of iron(iii)...
Questions


Social Studies, 09.10.2019 01:00





History, 09.10.2019 01:00









Mathematics, 09.10.2019 01:00




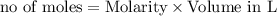
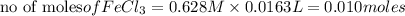
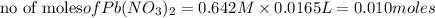
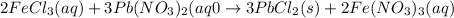
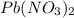 reacts with 2 moles of
reacts with 2 moles of 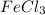
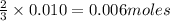 of
of 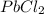
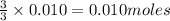 of
of