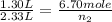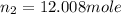Chemistry, 07.09.2019 03:10 allyssaharrisooy50au
Gaseous chlorine is held in two separate containers at identical temperature and pressure. the volume of container 1 is 1.30 l, and it contains 6.70 mol of the gas. the volume of container 2 is 2.33 l. how many moles of the gas are in container 2?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Gaseous chlorine is held in two separate containers at identical temperature and pressure. the volum...
Questions

Mathematics, 04.10.2020 21:01


Engineering, 04.10.2020 21:01


English, 04.10.2020 21:01

Chemistry, 04.10.2020 21:01



Mathematics, 04.10.2020 21:01

Mathematics, 04.10.2020 21:01

Computers and Technology, 04.10.2020 21:01


Mathematics, 04.10.2020 21:01




Biology, 04.10.2020 21:01


Mathematics, 04.10.2020 21:01

Mathematics, 04.10.2020 21:01

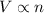
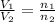
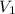 = initial volume of gas in container 1 = 1.30 L
= initial volume of gas in container 1 = 1.30 L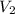 = final volume of gas in container 1 = 2.33 L
= final volume of gas in container 1 = 2.33 L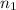 = initial moles of gas in container 2 = 6.70 mole
= initial moles of gas in container 2 = 6.70 mole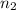 = final moles of gas in container 2 = ?
= final moles of gas in container 2 = ?