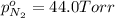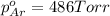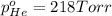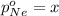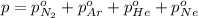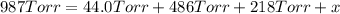Chemistry, 07.09.2019 03:30 rudondo4747
Avessel contained n2, ar, he, and ne. the total pressure in the vessel was 987 torr. the partial pressures of nitrogen, argon, and helium were 44.0, 486, and 218 torr, respectively. the partial pressure of neon in the vessel was torr. a) 42.4 b) 521 c) 19.4 d) 239 e) 760

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1


Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
Avessel contained n2, ar, he, and ne. the total pressure in the vessel was 987 torr. the partial pre...
Questions



Mathematics, 30.03.2021 19:20



English, 30.03.2021 19:20




Advanced Placement (AP), 30.03.2021 19:20


Mathematics, 30.03.2021 19:20



History, 30.03.2021 19:20



History, 30.03.2021 19:20

Mathematics, 30.03.2021 19:20

Mathematics, 30.03.2021 19:20