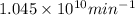Chemistry, 07.09.2019 04:20 COOLIOMARIS
Assuming that each atom that decays emits one alpha particle, how many alpha particles are emitted per minute by a 0.00456-g sample of products? the half-life of 47bk is 1.40x10°y and the mass of a 247bk atom is 247,070 u. that is free from its decay, alpha particles min submit answer 5 question attempts remaining

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 11:00
The decimals you found in part b are called repeating decimals. in the gizmo, repeating decimals are rounded to two places. how does the gizmo show you that a decimal has been rounded?
Answers: 3

Chemistry, 23.06.2019 11:00
Asolubility table shows that almost all compounds of group 1 metals are soluble in water. this general rule tells you that mgi2 is soluble rbno3 is soluble cacl2 is soluble co2 is soluble
Answers: 1

Chemistry, 23.06.2019 13:30
These traits describe either a chemical or a nuclear reaction. which statements describe a nuclear reaction? check all that apply. involves the loss, gain, or sharing of electrons may involve a change in total mass involve relatively low energy changes occur outside the nucleus involve very high-energy changes involve changes in nuclides when decay occurs
Answers: 1
You know the right answer?
Assuming that each atom that decays emits one alpha particle, how many alpha particles are emitted p...
Questions

Biology, 05.12.2019 11:31

Health, 05.12.2019 11:31

Mathematics, 05.12.2019 11:31

Spanish, 05.12.2019 11:31

Mathematics, 05.12.2019 11:31


History, 05.12.2019 11:31



Health, 05.12.2019 11:31




Mathematics, 05.12.2019 11:31

Social Studies, 05.12.2019 11:31



Mathematics, 05.12.2019 11:31

Biology, 05.12.2019 11:31

Biology, 05.12.2019 11:31

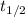 ) for
) for 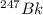 is
is 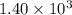 year
year 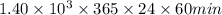 year
year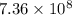 min
min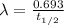
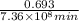
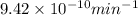
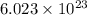 atoms of
atoms of 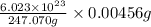
 atoms of
atoms of 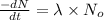
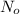 = no. of atoms present in given amount of substance
= no. of atoms present in given amount of substance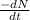 = 9.42 \times 10^{-10} min^{-1} \times 1.11 \times 10^{19}[/tex]
= 9.42 \times 10^{-10} min^{-1} \times 1.11 \times 10^{19}[/tex]