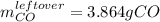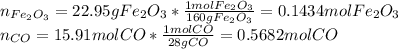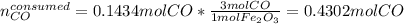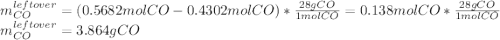Chemistry, 07.09.2019 04:30 TightKnowsDaWhey
Iron(iii) oxide reacts with carbon monoxide according to the equation: fe2o3(s) + 3 co(g) → 2 fe(s) + 3 co2(g) a reaction mixture initially contains 22.95 g fe2o3 and 15.91 g co. assume that the reaction will progress to 100% completion. what mass (in g) of the excess reactant is leftover?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1
You know the right answer?
Iron(iii) oxide reacts with carbon monoxide according to the equation: fe2o3(s) + 3 co(g) → 2 fe(s)...
Questions

Physics, 12.12.2020 15:50

Biology, 12.12.2020 15:50



History, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50


Health, 12.12.2020 15:50

Engineering, 12.12.2020 15:50



Chemistry, 12.12.2020 15:50

Health, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50

History, 12.12.2020 15:50

Chemistry, 12.12.2020 15:50

English, 12.12.2020 15:50