Chemistry, 07.09.2019 05:21 Lilleypad07
A0.418 g sample of kcl is added to 35.2 g of water in a calorimeter. if the temperature decreases by 1.01°c, what is the approximate amount of heat (in j) involved in the dissolution of the kcl, assuming the heat capacity of the resulting solution is 4.18 j/g°c? j is the reaction exothermic or endothermic?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
A0.418 g sample of kcl is added to 35.2 g of water in a calorimeter. if the temperature decreases by...
Questions






History, 10.03.2020 12:14

Mathematics, 10.03.2020 12:17

Chemistry, 10.03.2020 12:18

Advanced Placement (AP), 10.03.2020 12:19


History, 10.03.2020 13:21

Mathematics, 10.03.2020 13:21

Mathematics, 10.03.2020 13:23

Chemistry, 10.03.2020 13:23




English, 10.03.2020 13:26


Biology, 10.03.2020 13:26

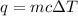
 = 1.01 °C
= 1.01 °C