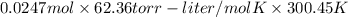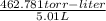Chemistry, 07.09.2019 05:30 dogsarecute278
The vapor pressure of a volatile liquid can be determined by slowly bubbling a known volume of gas through it at a known temperature and pressure. in an experiment, 5.01 l of n2 gas is passed through 7.9286 g of liquid benzene, c6h6, at 27.3 ∘c and atmospheric pressure. the liquid remaining after the experiment weighs 5.9987 g . assuming that the gas becomes saturated with benzene vapor and that the total gas volume and temperature remain constant, what is the vapor pressure of the benzene in torr?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
You know the right answer?
The vapor pressure of a volatile liquid can be determined by slowly bubbling a known volume of gas t...
Questions

World Languages, 03.08.2019 16:30

Chemistry, 03.08.2019 16:30

Mathematics, 03.08.2019 16:30



History, 03.08.2019 16:30


Geography, 03.08.2019 16:30

Computers and Technology, 03.08.2019 16:30





Mathematics, 03.08.2019 16:30


World Languages, 03.08.2019 16:30

Computers and Technology, 03.08.2019 16:30


Mathematics, 03.08.2019 16:30

Mathematics, 03.08.2019 16:30

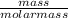
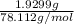
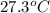 = 27.3 + 273.15 = 300.45 K
= 27.3 + 273.15 = 300.45 K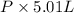 =
=