
Chemistry, 09.09.2019 00:20 Greghairston9813
The radius of a uranium atom is 149 pm. how many uranium atoms would have to be laid side by side to span a distance of 4.96 mm?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
The radius of a uranium atom is 149 pm. how many uranium atoms would have to be laid side by side to...
Questions




Mathematics, 06.04.2021 19:30




Biology, 06.04.2021 19:30


Biology, 06.04.2021 19:30

English, 06.04.2021 19:30

Mathematics, 06.04.2021 19:30

English, 06.04.2021 19:30

Mathematics, 06.04.2021 19:30




Mathematics, 06.04.2021 19:30


Biology, 06.04.2021 19:30

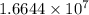 atoms.
atoms.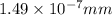
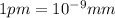
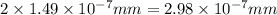
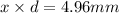
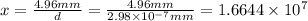 atoms.
atoms.

