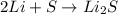The chemical equation given below represents the chemical reaction between lithium (li) and sulfur (s). in the equation, why is the number 2 present in front of lithium on the reactants side of the equation?
a. to show the number of lithium atoms involved in the reaction
b. to show the number of electrons gained by lithium in the reaction
c. to show the atomic number of lithium in the periodic table
d. to show the number of electrons lost by lithium in the reaction

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
The chemical equation given below represents the chemical reaction between lithium (li) and sulfur (...
Questions

English, 06.11.2020 09:40

History, 06.11.2020 09:40

English, 06.11.2020 09:40

Mathematics, 06.11.2020 09:40

Chemistry, 06.11.2020 09:40

Law, 06.11.2020 09:40


English, 06.11.2020 09:40


Mathematics, 06.11.2020 09:40


Mathematics, 06.11.2020 09:40


Mathematics, 06.11.2020 09:40



Mathematics, 06.11.2020 09:40

Mathematics, 06.11.2020 09:40


English, 06.11.2020 09:40