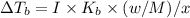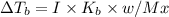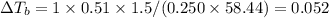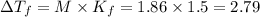Chemistry, 09.09.2019 06:10 bradydodson47
What are deltatb and deltatf for an aqueous solution that is 1.5g nacl in 0.250kg h2o? given kb=0.51 c/m and kr=1.86 c/m

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3


Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
What are deltatb and deltatf for an aqueous solution that is 1.5g nacl in 0.250kg h2o? given kb=0.5...
Questions

History, 23.06.2019 23:40

Mathematics, 23.06.2019 23:40


Spanish, 23.06.2019 23:40

History, 23.06.2019 23:40

Biology, 23.06.2019 23:40








Mathematics, 23.06.2019 23:40

Mathematics, 23.06.2019 23:40

History, 23.06.2019 23:40




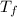 for given question is 2.79 and
for given question is 2.79 and 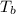 is 0.52
is 0.52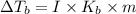 {i- vant hoff’s constant ; Kb- constant ; m molarity }
{i- vant hoff’s constant ; Kb- constant ; m molarity }