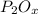An unknown compound has the following chemical formula:
p_2o_x
where x stands for a who...

An unknown compound has the following chemical formula:
p_2o_x
where x stands for a whole number.
measurements also show that a certain sample of the unknown compound contains 8.20 mol of oxygen and 3.3mol of phosphorus.
write the complete chemical formula for the unknown compound.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

You know the right answer?
Questions







Mathematics, 23.05.2020 05:04

Computers and Technology, 23.05.2020 05:04





English, 23.05.2020 05:04



Mathematics, 23.05.2020 05:04



English, 23.05.2020 05:04