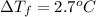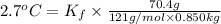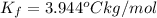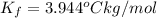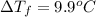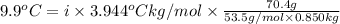Chemistry, 09.09.2019 18:30 chloejaylevesque
When 70.4 g of benzamide (c, h,no) are dissolved in 850. g of a certain mystery liquid x, the freezing point of the solution is 2.7 c lower than the freezing point of pure x. on the other hand, when 70.4 g of ammonium chloride (nh ci) are dissolved in the same mass of x, the freezing point of the solution is 9.9 °c lower than the freezing point of pure x. calculate the van't hoff factor for ammonium chloride in x. be sure your answer has a unit symbol, if necessary, and round your answer to 2 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3


Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
When 70.4 g of benzamide (c, h,no) are dissolved in 850. g of a certain mystery liquid x, the freezi...
Questions


History, 02.02.2021 22:30


Mathematics, 02.02.2021 22:30



Mathematics, 02.02.2021 22:30

Social Studies, 02.02.2021 22:30

Arts, 02.02.2021 22:30

Mathematics, 02.02.2021 22:30


Health, 02.02.2021 22:30

Social Studies, 02.02.2021 22:30




Mathematics, 02.02.2021 22:30

Chemistry, 02.02.2021 22:30

Mathematics, 02.02.2021 22:30

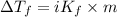
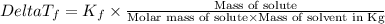 ...(1)
...(1)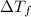 =Elevation in boiling point =
=Elevation in boiling point = 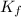 = Freezing point constant
= Freezing point constant