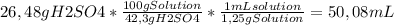Chemistry, 09.09.2019 19:10 runaway173
50 ml of a mixture consisting of 0.019 m cerium (iv) and 2.7 m h2so4, or sulfuric acid. begin by preparing 100 ml of 2.7 m h2so4 using the available solution of 42.3% w/w h2so4. this concentration unit, which may be less familiar to you, is a weight-to-weight percent (100.0 g of the solution contains 42.3 g of h2so4). the density of 42.3% w/w h2so4 is 1.25 g solution/ml solution. using a graduated cylinder, measure out the correct volume of 42.3% w/w h2so4 and slowly add it to a 100-ml volumetric flask that already contains approximately 25 ml of deionized water.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2


Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
50 ml of a mixture consisting of 0.019 m cerium (iv) and 2.7 m h2so4, or sulfuric acid. begin by pre...
Questions



Biology, 26.02.2020 19:32




English, 26.02.2020 19:32

History, 26.02.2020 19:32










Biology, 26.02.2020 19:32

Mathematics, 26.02.2020 19:32