
Chemistry, 09.09.2019 19:30 reaperdraws
Which of the following solutes will lower the freezing point of water the most?
a.
the molecular compound sucrose (c12h22o11)
b.
the ionic compound magnesium sulfate (mgso4)
c.
the ionic compound lithium chloride (licl)
d.
the ionic compound calcium fluoride (caf2)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1
You know the right answer?
Which of the following solutes will lower the freezing point of water the most?
a.
the...
a.
the...
Questions


English, 13.10.2020 06:01

Computers and Technology, 13.10.2020 06:01



Biology, 13.10.2020 06:01

Mathematics, 13.10.2020 06:01


Social Studies, 13.10.2020 06:01


Social Studies, 13.10.2020 06:01



Geography, 13.10.2020 06:01




Chemistry, 13.10.2020 06:01


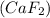
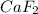 and the freezing point will be lowest.
and the freezing point will be lowest.


