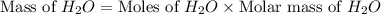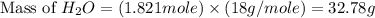Chemistry, 09.09.2019 19:30 Frenchfries13
Consider the following unbalanced chemical equation. c5h12(l) + o2(g) → co2(g) + h2o(l) if 21.9 grams of pentane (c5h12) are burned in excess oxygen, how many grams of h2o will be produced?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
Consider the following unbalanced chemical equation. c5h12(l) + o2(g) → co2(g) + h2o(l) if 21.9 gram...
Questions






Physics, 11.07.2019 20:30


Mathematics, 11.07.2019 20:30


Advanced Placement (AP), 11.07.2019 20:30

History, 11.07.2019 20:30



Mathematics, 11.07.2019 20:30

Mathematics, 11.07.2019 20:30

Chemistry, 11.07.2019 20:30

Social Studies, 11.07.2019 20:30

Mathematics, 11.07.2019 20:30

Mathematics, 11.07.2019 20:30

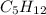 = 21.9 g
= 21.9 g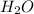 = 18 g/mole
= 18 g/mole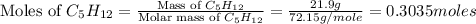
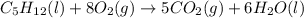
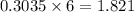 moles of
moles of