
Chemistry, 09.09.2019 22:10 fiorentinologan4
The haber-bosch process is a very important industrial process. in the haber-bosch process, hydrogen gas reacts with nitrogen gas to produce ammonia according to the equation 3h2(g)+n2(g)→2nh3(g) the ammonia produced in the haber-bosch process has a wide range of uses, from fertilizer to pharmaceuticals. however, the production of ammonia is difficult, resulting in lower yields than those predicted from the chemical equation. 1.15 g h2 is allowed to react with 9.93 g n2, producing 1.12 g nh3. part a what is the theoretical yield in grams for this reaction under the given conditions? express your answer to three significant figures and include the appropriate units.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2


Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
You know the right answer?
The haber-bosch process is a very important industrial process. in the haber-bosch process, hydrogen...
Questions

Mathematics, 06.11.2020 02:50

Mathematics, 06.11.2020 02:50

Mathematics, 06.11.2020 02:50

Mathematics, 06.11.2020 02:50

Biology, 06.11.2020 02:50













Mathematics, 06.11.2020 02:50

Mathematics, 06.11.2020 02:50

Biology, 06.11.2020 02:50

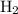 and
and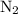 .
.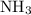 will be produced?
will be produced?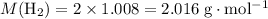 .
.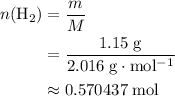 .
.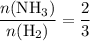 .
.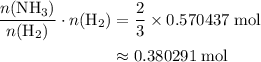 .
.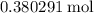 of
of 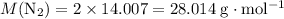 .
.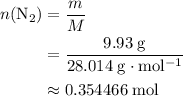 .
.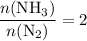 .
.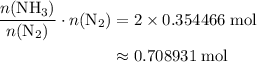 .
.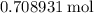 of
of 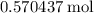 of
of 

