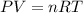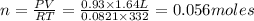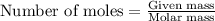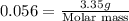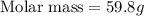Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1
You know the right answer?
A3.35 gram sample of an unknown gas is found to occupy a volume of 1.64 l at a pressure of 706 mmhg...
Questions



Mathematics, 24.09.2020 18:01

Computers and Technology, 24.09.2020 18:01

Biology, 24.09.2020 18:01

Mathematics, 24.09.2020 18:01



Business, 24.09.2020 18:01


Mathematics, 24.09.2020 18:01

Mathematics, 24.09.2020 18:01

Geography, 24.09.2020 18:01


Mathematics, 24.09.2020 18:01


Chemistry, 24.09.2020 18:01

Mathematics, 24.09.2020 18:01