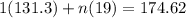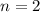Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1
You know the right answer?
Agiven sample of a xenon fluoride compound contains molecules of a single type xefn, where n is some...
Questions



History, 06.05.2021 07:00

Mathematics, 06.05.2021 07:00




Mathematics, 06.05.2021 07:00


Mathematics, 06.05.2021 07:00

Mathematics, 06.05.2021 07:00


Advanced Placement (AP), 06.05.2021 07:00



Mathematics, 06.05.2021 07:00




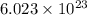 of particles.
of particles.
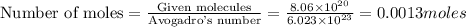
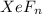 weigh = 0.227 grams
weigh = 0.227 grams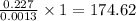 grams
grams