
Chemistry, 10.09.2019 18:30 mikayleighb2019
Why does ammonium nitrate (nh4no3) dissolve readily in water even though the dissolution process is endothermic by 26.4 kj/mol? why does ammonium nitrate (nh4no3) dissolve readily in water even though the dissolution process is endothermic by 26.4 kj/mol? the vapor pressure of the water decreases upon addition of the solute. the osmotic properties of the system lead to this behavior. the overall enthalpy of the system decreases upon addition of the solute. the overall entropy of the system increases upon dissolution of this strong electrolyte. the overall enthalpy of the system increases upon dissolution of this strong electrolyte.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

You know the right answer?
Why does ammonium nitrate (nh4no3) dissolve readily in water even though the dissolution process is...
Questions







Mathematics, 27.04.2021 20:10

Computers and Technology, 27.04.2021 20:10

Mathematics, 27.04.2021 20:10

Mathematics, 27.04.2021 20:10



History, 27.04.2021 20:10


Chemistry, 27.04.2021 20:10



Chemistry, 27.04.2021 20:10


English, 27.04.2021 20:10

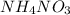 is ionic in nature. Hence, when it is added to water then it will readily dissociate into ammonium ions (
is ionic in nature. Hence, when it is added to water then it will readily dissociate into ammonium ions (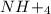 ) and nitrate ions (
) and nitrate ions (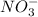 ).
).

