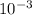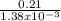The partial pressure of o2 in air at sea level is 0.21atm. the solubility of o2 in water at 20∘c, with 1 atm o2 pressure is 1.38×10−3 m. part a using henry's law, calculate the molar concentration of o2 in the surface water of a mountain lake saturated with air at 20 ∘c and an atmospheric pressure of 665 torr . express your answer using two significant figures. nothing

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2


Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
The partial pressure of o2 in air at sea level is 0.21atm. the solubility of o2 in water at 20∘c, wi...
Questions

Physics, 10.12.2020 04:40

English, 10.12.2020 04:40

Mathematics, 10.12.2020 04:40

Mathematics, 10.12.2020 04:40



Mathematics, 10.12.2020 04:40

Mathematics, 10.12.2020 04:40

Mathematics, 10.12.2020 04:40

Computers and Technology, 10.12.2020 04:40

Physics, 10.12.2020 04:40


History, 10.12.2020 04:40


Mathematics, 10.12.2020 04:40


History, 10.12.2020 04:40



Mathematics, 10.12.2020 04:40

 M
M