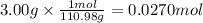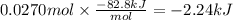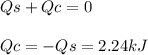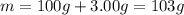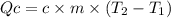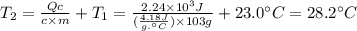Chemistry, 10.09.2019 19:30 santos200154
In the following experiment, a coffee-cup calorimeter containing 100 ml of h2o is used. the initial temperature of the calorimeter is 23.0 ∘c. if 3.00 g of cacl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? the heat of solution δhsoln of cacl2 is −82.8 kj/mol. assume that the specific heat of the solution form

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 09:00
Individuals within populations exhibit some diversity. as a result of possessing slightly different traits, some individuals are better able to survive and reproduce than others. if these individuals changes in the characteristics of the population may occur over time. the cumulative change in these characteristics is known as
Answers: 3

Chemistry, 23.06.2019 09:20
Which of the following occurs along coasts during the day?
Answers: 3
You know the right answer?
In the following experiment, a coffee-cup calorimeter containing 100 ml of h2o is used. the initial...
Questions


Social Studies, 05.02.2020 01:52





History, 05.02.2020 01:52


Business, 05.02.2020 01:52

Geography, 05.02.2020 01:52

Mathematics, 05.02.2020 01:52


Health, 05.02.2020 01:52

History, 05.02.2020 01:52


Computers and Technology, 05.02.2020 01:52



History, 05.02.2020 01:52

Mathematics, 05.02.2020 01:52