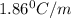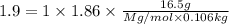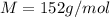Chemistry, 10.09.2019 23:10 blakestuhan
An aqueous solution containing 16.5 g of an unknown molecular (nonelectrolyte) compound in 106.0 g of water was found to have a freezing point of -1.9 ∘c. calculate the molar mass of the unknown compound.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:50
It has been two weeks since charles met with daniel, a dietitian, who provided charles with a menu for weight loss. charles and his mother are going back to see daniel again with a chart of the food charles has eaten. the following lists what charles ate in one day: breakfast 1 banana, 1 cup of nonfat milk, 1 egg lunch 1 cup of carrots, 3 oz of steak, 1 apple, 1 cup of nonfat milk dinner 6 oz of skinless chicken, 1 baked potato, 3 oz of broccoli, 1 cup of nonfat milk
Answers: 1

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

You know the right answer?
An aqueous solution containing 16.5 g of an unknown molecular (nonelectrolyte) compound in 106.0 g o...
Questions

English, 16.10.2019 11:00




Computers and Technology, 16.10.2019 11:00


Biology, 16.10.2019 11:00


History, 16.10.2019 11:00

Biology, 16.10.2019 11:00

English, 16.10.2019 11:00

Social Studies, 16.10.2019 11:00

Mathematics, 16.10.2019 11:00

Mathematics, 16.10.2019 11:00

Biology, 16.10.2019 11:00

Computers and Technology, 16.10.2019 11:00

Mathematics, 16.10.2019 11:00


Mathematics, 16.10.2019 11:00

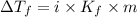
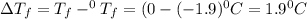 = Depression in freezing point
= Depression in freezing point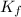 = freezing point constant =
= freezing point constant =