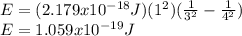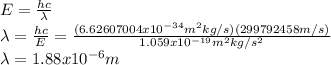What is the least amount of energy that can be emitted by an excited electron in a hydrogen atom falling from an excited state directly to the n = 3 state? what is the quantum number n for the excited state? humans cannot visually observe the photons emitted in this process. why not?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3
You know the right answer?
What is the least amount of energy that can be emitted by an excited electron in a hydrogen atom fal...
Questions

Physics, 21.03.2021 03:00


Mathematics, 21.03.2021 03:00


Spanish, 21.03.2021 03:00



Mathematics, 21.03.2021 03:00

Mathematics, 21.03.2021 03:00

Mathematics, 21.03.2021 03:00




Advanced Placement (AP), 21.03.2021 03:00

Chemistry, 21.03.2021 03:00


Mathematics, 21.03.2021 03:00


Mathematics, 21.03.2021 03:00

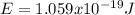
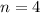
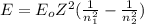
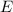 is the emitted energy,
is the emitted energy, 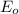 the first level energy,
the first level energy, 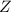 the atomic number,
the atomic number, 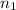 the first level and
the first level and 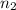 the second level. In such a way, the closest the
the second level. In such a way, the closest the 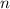 's are, the least the amount of emitted energy, therefore,
's are, the least the amount of emitted energy, therefore, 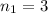 (based on the statement) and
(based on the statement) and 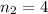 , thus, the least amount of energy turns out being:
, thus, the least amount of energy turns out being: