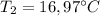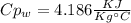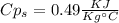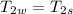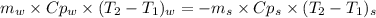Chemistry, 11.09.2019 00:30 sedratkawaiah13
A1.28-kg sample of water at 10.0 °c is in a calorimeter. you drop a piece of steel with a mass of 0.385 kg at 215 °c into it. after the sizzling subsides, what is the final equilibrium temperature? (make the reasonable assumptions that any steam produced condenses into liquid water during the process of equilibration and that the evaporation and condensation don’t affect the outcome, as we’ll see in the next section.)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

You know the right answer?
A1.28-kg sample of water at 10.0 °c is in a calorimeter. you drop a piece of steel with a mass of 0....
Questions


Physics, 20.04.2021 21:40

Mathematics, 20.04.2021 21:40

Mathematics, 20.04.2021 21:40


Mathematics, 20.04.2021 21:40

English, 20.04.2021 21:40

Mathematics, 20.04.2021 21:40

Mathematics, 20.04.2021 21:40


Chemistry, 20.04.2021 21:40




Mathematics, 20.04.2021 21:40

Mathematics, 20.04.2021 21:40




History, 20.04.2021 21:40